我们在准备IGCSE化学考试过程中,知识点的掌握是基础,也是最重要的一部分。为了帮助同学们做好化学复习工作,A加未来小编就为大家整理了Paper2部分的一些重要的IGCSE化学考点,知识点还不够扎实的同学们可以借此机会做好查漏补缺的工作。
这些是考试重点内容:
atomic structure
reactivity of metals
chemical test
organic
acid base and salt
calculation
energy change
bond energy
chromatography
elements,compounds and mixtures
the periodic table
rate of reaction
electrolysis
redox
equilibrium
gas in atmosphere
group 7
state of matter
group 1
titration
dot and cross digram
bonding and structure
crude oil
ammonia
formula equation
group 2
alkene
今天,我主要来说说chemical test部分。按照大纲要求:
(1)describe tests for these gases:
•hydrogen【‘pops’with a lighted splint】
•oxygen【relights a glowing splint】
•carbon dioxide【gives a white ppt.with limewater】
•ammonia【turns damp red litmus paper blue】
•chlorine【bleaches damp litmus paper】
(2)describe how to carry out a flame test
【1.The technique is first of all to clean the end of a piece of platinum or nichrome wire by dipping it into clean hydrochloric acid and then placing it in a roaring Bunsen flame.This procedure should be repeated until the wire no longer produces a colour in the flame.
2.The end of the wire should then be dipped into fresh hydrochloric acid and then into the solid sample under test.
3.The end of the wire should then be placed into a non-roaring,non-luminous Bunsen flame.】
(3)know the colours formed in flame tests for these cations:
•Li+is red
•Na+is yellow
•K+is lilac
•Ca2+is orange-red
•Cu2+is blue-green
(4)test of cations
•NH4+using sodium hydroxide solution and identifying the gas evolved【放出的气体是NH3】
•Cu2+,Fe2+and Fe3+using sodium hydroxide solution【Cu(OH)2,Fe(OH)2,Fe(OH)3颜色分别是blue,green,red-brown】
(5)test of anions
•Cl–,Br–and I–using acidified silver nitrate solution【AgCl,AgBr,AgI颜色分别是white,cream,yellow】
•SO42–using acidified barium chloride solution【BaSO4是white precipitate】
•CO32–using hydrochloric acid and identifying the gas evolved.【CO2 is released】
来,我们看道真题:
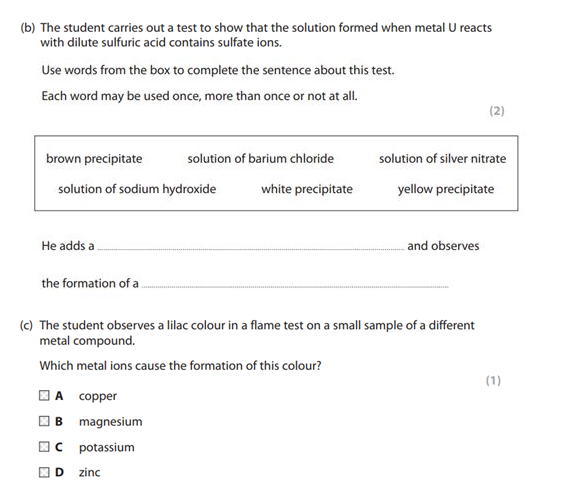
解析:b题,含有SO42-离子的检验,那使用含有Ba2+离子的物质检验,答案选择【solution of barium chloride】,观察到白色沉淀BaSO4,因此答案是【white precipitate】。
C问,flame test中是lilac颜色的metal ion是K,答案选择potassium.
怎么样,对于这部分的IGCSE化学考点你是不是已经深刻的掌握了呢?如果在学习过程中还有什么疑问,欢迎随时咨询我们的在线老师,让老师一对一为你进行专业的课程学习辅导吧!